EC Declaration of Conformity & CE mark
In order to certify that machinery conforms to the requirements of the MD, the manufacturer must issue an EC Declaration of Conformity for every machine produced.
It is the manufacturer who attaches the CE mark to operational machinery, and not notified testing laboratories such as the TÜV.
The CE mark is not a seal of quality!
Rather, it should be viewed as a kind of passport which, when attached to machinery, both confirms that the manufacturer has ensured compliance with the MD and enables the machinery to cross national borders within the EU.
Procedure for obtaining the CE mark
1. Complying with the essential health and safety requirements laid down in Annex I of the MD
2. Observing all other relevant directives
3. Compiling technical documentation
4. EC prototype testing, where necessary
5. Issuing a Declaration of Conformity
6. Attaching the CE mark
Area of application of the new MD
The Directive applies to the following products:
• Machines
• Interchangeable equipment
• Safety components
• Lifting accessories
• Chains, ropes and webbing used for lifting
• Removable mechanical transmission devices
• Partly completed machinery
“Partly completed machine”
This is defined as a single entity that almost constitutes a machine, and can be taken as an entity in and of itself, but is unable to perform a specific function by itself.
A drive system is a partly completed machine. A partly completed machine is only intended to be incorporated into or added to other machines, other partly completed machines, or equipment, for the purpose of forming a machine (within the sense of this Directive) with them.
Changes for “partly completed machinery”
Requirements for “part machinery” (also known as “partly completed machinery”) have been re-established in the amended version of the Machinery Directive.
In cases where a manufacturer’s declaration was sufficient in the past, the manufacturer will in future need to supply a declaration of incorporation. This must specify the requirements of the Directive that affect the part machine in question and those that have been adhered to.
Installation instructions must be supplied with the documentation for the machine.
Essential health and safety requirements
Now, the essential health and safety requirements (Annex I) will require the manufacturer to carry out a risk assessment (see EN ISO 12100).
Other changes affect requirements in areas such as:
• Ergonomics
• Control systems and protective devices
• Noise and vibration emissions
CASE STUDIES
August 2018
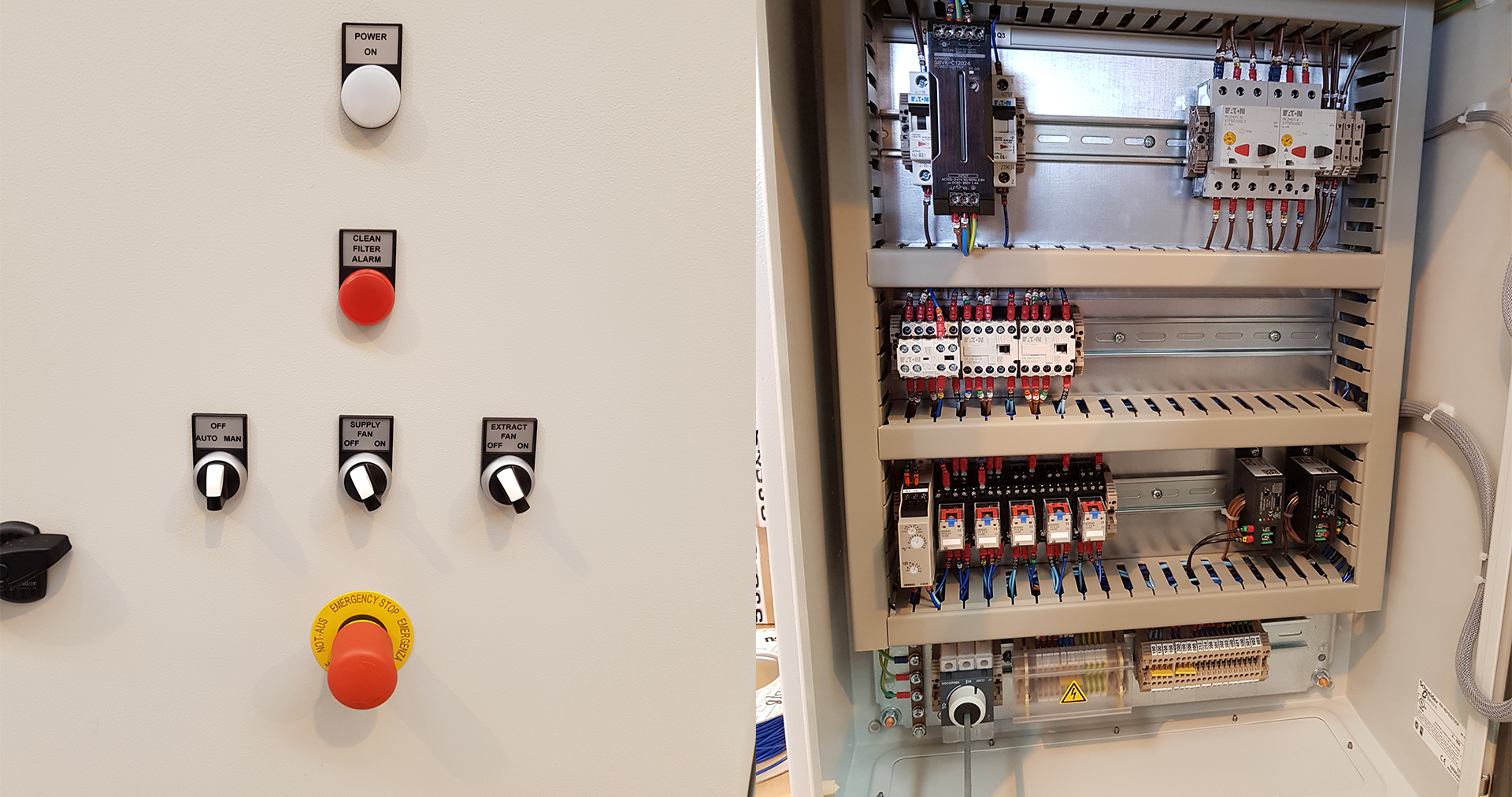
Ventilation System
Project Description APS have just designed and built some new [...]
April 2018
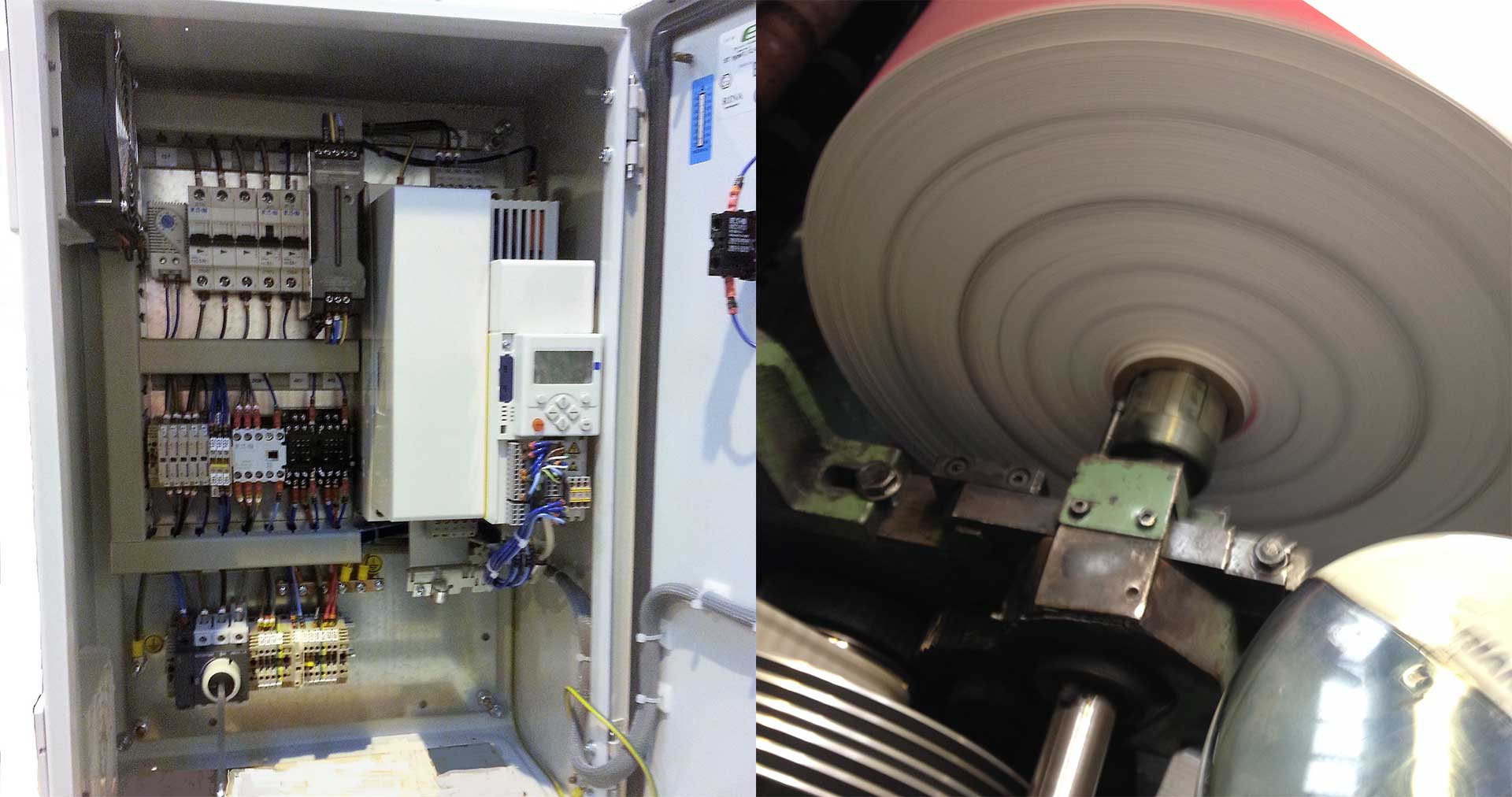
Winding Control
Project Description David Emmett, the owner of British Paper Coils [...]
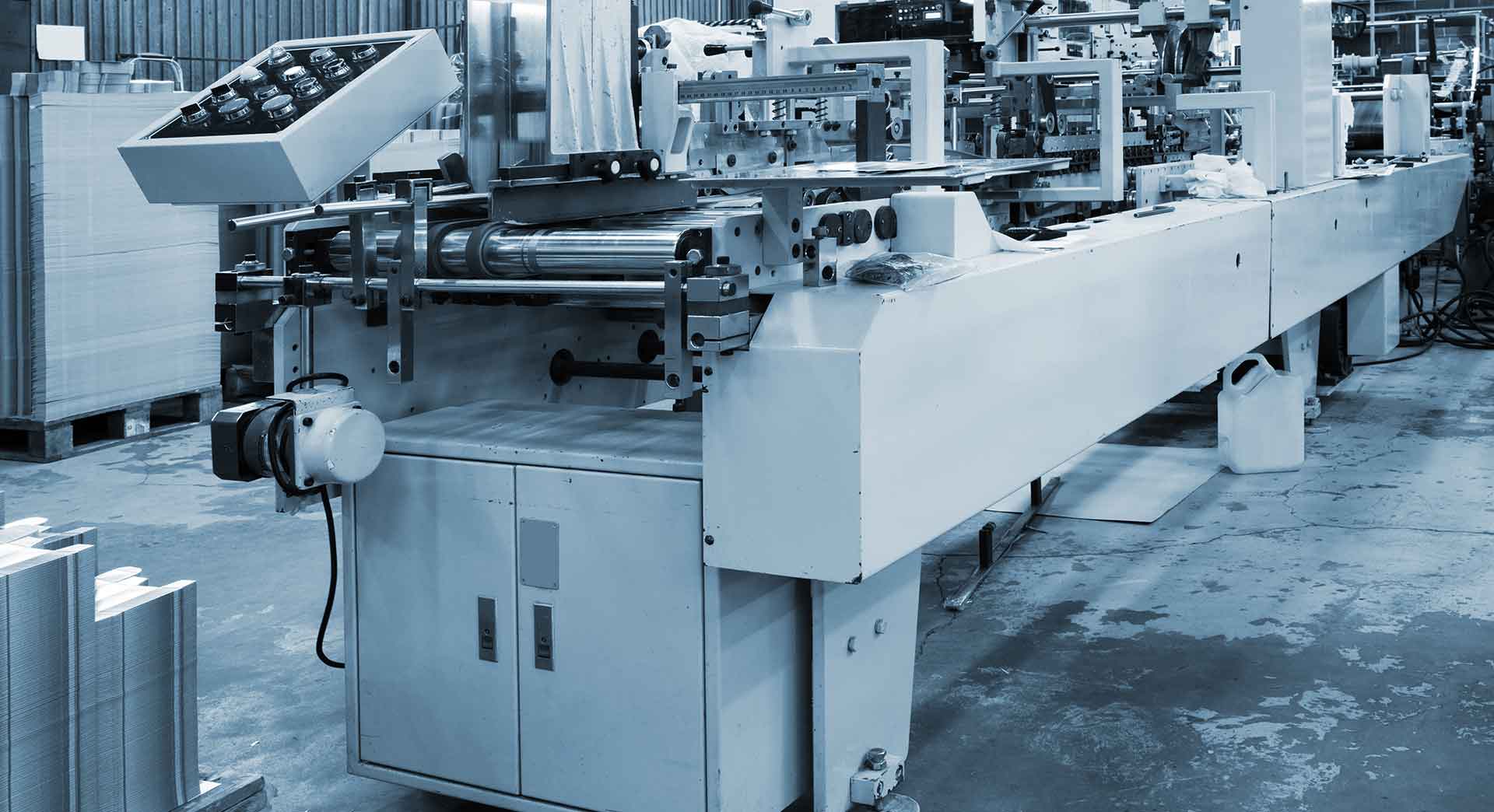
PRINTING INDUSTRY
Printing Industry APS (Automation Products and Systems) have provided systems [...]
 If you are looking for an experienced product automation specialist or help with installtion, service or support then please fill in your details below and we will get right back to you.
If you are looking for an experienced product automation specialist or help with installtion, service or support then please fill in your details below and we will get right back to you.